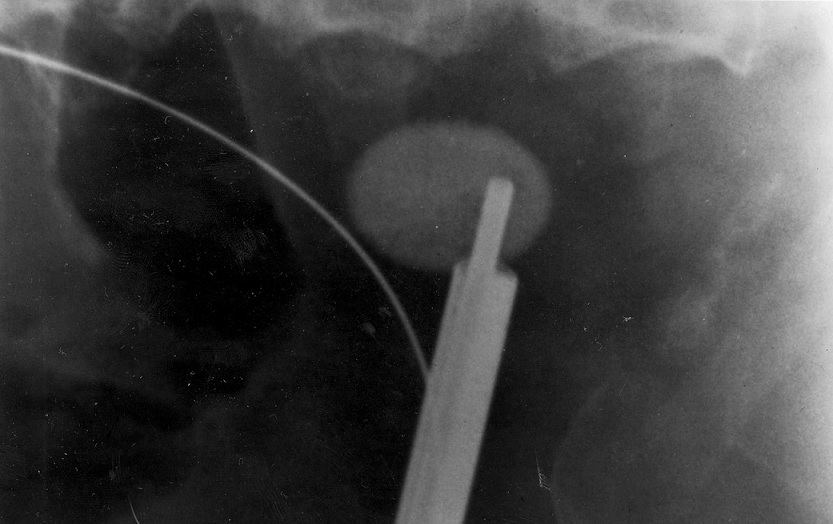
A team of staff from ITE has successfully created a solution to simplify percutaneous-nephrolithotomy (PCNL), which is the complicated process of removing large or complex kidney stones. The Percutaneous-Access-to-Kidney-Assist Device (PAKAD) is made possible through ITE’s collaboration with the National University Hospital (NUH), the National University of Singapore (NUS) and Invivo Medical Pte Ltd.
Innovation to Make Complex Procedure Simpler, Shorter and Safer
PCNL is a complicated surgery technique that is usually carried out by senior surgeons. Traditionally, PCNL surgeons refer to x-ray imaging of the patient’s kidney to locate the stone(s), before inserting a long and hollow needle through the skin to reach the stone(s). An endoscope surgical instrument is then inserted through the aid of the needle passage to fragment and remove the stone(s). This is complex, as the surgeon has to use free-hand techniques to locate the stone(s) in a three-dimensional environment, while referring to a two-dimensional image. At times, several attempts may be required, which lengthens the patient’s exposure to x-ray radiation and recovery period.
The PAKAD incorporates precision engineering mechanisms to systematically adjust, guide and stabilize the needle into alignment with the targeted stone(s). Through successful trials on animals at NUH, the PAKAD has proven that it can simplify and shorten the PCNL process, hence reducing x-ray exposure, risks of complications, and recovery periods. With the PAKAD, the junior surgeons can also perform the needle insertion PCNL procedure
Multiple Parties Make Innovation Possible
ITE’s team’s research efforts were strengthened through the domain expertise of NUH and NUS. Through Invivo Medical, the device will be commercialized and a licensing agreement was signed among ITE, NUH, NUS and Invivo Medical. The team received financial support for the Applied Research through the MOE Innovation Fund, the NRF Proof-of-Concept grant, and the MOE Translational R&D and Innovation Fund.
Work on the PAKAD started in 2011. After the PAKAD was developed and a patent application was filed, the PAKAD was first showcased at a major event at TechInnovation 2013, as part of the Singapore start-up ecosystem. At the event, PAKAD was matched with an investor, who is now bringing the device to market through Invivo Medical. Over the next five years, the sales of the device is projected to hit S$25 million.
The contributions by various parties have made it possible for the innovation to benefit patients. The research team will complete clinical trials before obtaining regulatory approvals. PAKAD is expected to reach the market by the end of 2018.
“This is an excellent example of an industry-institution partnership to develop innovative and integrated solutions to improve productivity and processes. We are grateful to NUH, NUS and Invivo Medical, to enable ITE to create and patent the Percutaneous Access to Kidney Assist Device (PAKAD) for safe kidney surgery,” said Ms Low Khah Gek, CEO, ITE.
“The new technology allows for better precision during surgery and enhances patient safety and outcome. It expands on NUH’s track record of partnerships for clinical innovation and development that help raise the standard of clinical care. We look forward to more of these collaborations to bring about greater tangible benefits for our patients,” Prof Kesavan Esuvaranathan, Head and Senior Consultant, Department of Urology, National University Hospital.
“NUS is delighted to have been part of this multi-party collaboration. Researchers from the NUS Yong Loo Lin School of Medicine provided medical expertise, which ITE used to enhance the PAKAD. Realising the strong potential for this technology to improve clinical procedures and healthcare for kidney stone patients, the NUS Industry Liaison Office took the lead in the commercialization process, including managing negotiations for this multi-party licensing agreement. Our endeavor to bring innovative technologies closer to market is made possible with great partners who have similar goals,” said Mr Sean Flanigan, Director of NUS Industry Liaison Office.
“The new medical-device is the world’s first operational Percutaneous Access to Kidney Assist Device (PAKAD). It is the result of a successful collaboration by three institutions and the industry and made possible by a match-up at TechInnovation 2013. Beyond this, we plan to do continual product development to apply the invention to more minimally-invasive surgical and biopsy procedures,” said Dr Joseph Chai, Managing Director / Director, Invivo Medical Pte Ltd.
Source: https://biotechin.asia